Resveratrol's Molecular Symphony: How a Wine Compound Orchestrates Cellular Anti-Aging
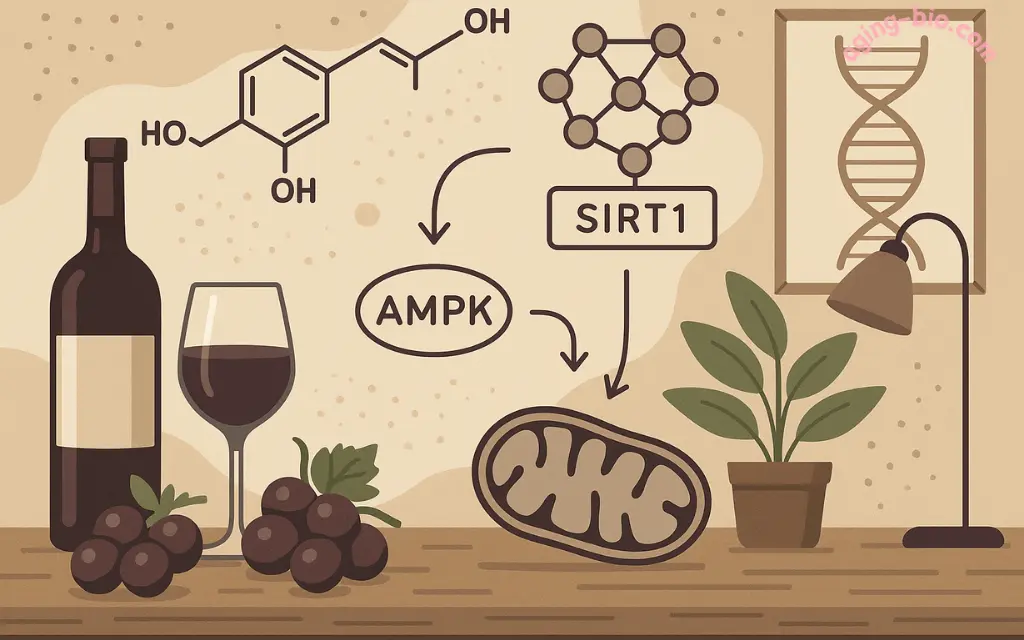
In 2003, when Dr. David Sinclair observed yeast cells living 70% longer after exposure to a molecule found in red wine, he was witnessing one of biology's most sophisticated molecular orchestrations. That molecule—resveratrol—wasn't simply "activating longevity genes." It was conducting an entire cellular symphony involving dozens of proteins, multiple pathways, and intricate molecular cascades that would revolutionize our understanding of aging.
What appeared to be a simple discovery has revealed itself as a masterclass in cellular biology. Resveratrol doesn't just flip a single anti-aging switch—it simultaneously activates protective pathways, enhances cellular powerhouses, suppresses damaging processes, and promotes repair mechanisms through an elegant network of molecular interactions that scientists are still uncovering today.
The Evolutionary Foundation: Xenohormesis and Molecular Stress Signals
Plant Stress as a Molecular Beacon
Resveratrol's story begins with evolutionary biology. When grapevines face environmental stress—fungal attacks, UV radiation, drought—they dramatically increase resveratrol production. This stilbene compound serves as more than just plant protection; it's a sophisticated molecular distress signal that concentrates in grape skins as an SOS broadcast to the environment.
Dr. Sinclair termed this phenomenon "xenohormesis"—the evolutionary strategy where animals benefit from sensing stress signals produced by other species. When animals consume plants containing stress-induced compounds like resveratrol, they activate their own cellular defense systems. This creates a remarkable biological communication system where plant stress becomes animal protection.
This evolutionary perspective explains why resveratrol and similar compounds are so potent: they're molecular messages that have been refined over millions of years to trigger robust cellular responses. The very mechanisms that help plants survive environmental challenges have been co-opted by animal cells to enhance their own survival and longevity.
The Biochemical Discovery Revolution
The breakthrough came when Konrad Howitz developed a biochemical screening system to identify sirtuin activators. When resveratrol entered this assay, it demonstrated unprecedented potency—activating SIRT1 with 13 times greater efficiency than any previously known compound.
This discovery was revolutionary because sirtuins had already been linked to the beneficial effects of caloric restriction, the only proven method for extending mammalian lifespan. For the first time, researchers had identified a compound that could pharmacologically activate the same longevity pathways triggered by dietary restriction, but without requiring reduced food intake.
SIRT1: The Master Molecular Conductor
Allosteric Activation: A Sophisticated Molecular Dance
Resveratrol's interaction with SIRT1 represents one of the most elegant examples of allosteric enzyme regulation in biology. Rather than simply binding to SIRT1's active site, resveratrol binds to a separate allosteric site, causing a conformational change that dramatically enhances the enzyme's activity.
Recent FRET-based studies have confirmed that this activation involves an amino terminal domain near SIRT1's catalytic core. The binding creates a structural change that increases SIRT1's affinity for both its NAD+ cofactor and protein substrates. This mechanism explains why resveratrol can activate SIRT1 with native substrates in vivo, resolving earlier controversies about fluorescent assay artifacts.
NAD+ Dependency: The Energy-Longevity Connection
SIRT1's absolute requirement for NAD+ as a cofactor creates a direct molecular link between cellular energy status and longevity pathways. This dependency means SIRT1 activity fluctuates with the cell's energy state:
- High Energy States: When cells are energy-replete, NAD+ levels drop relative to NADH, reducing SIRT1 activity
- Energy Stress: During fasting or exercise, increased NAD+/NADH ratios activate SIRT1, triggering protective responses
- Resveratrol Enhancement: By increasing SIRT1's catalytic efficiency, resveratrol amplifies this natural energy-sensing system
This molecular mechanism explains why resveratrol can mimic many benefits of caloric restriction—it enhances the same energy-sensing pathway that dietary restriction naturally activates.
SIRT1's Molecular Target Network
The breadth of SIRT1's molecular targets explains resveratrol's wide-ranging anti-aging effects:
PGC-1α: The Mitochondrial Master Switch When SIRT1 deacetylates PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), it transforms this protein into the cell's most powerful signal for mitochondrial enhancement. Deacetylated PGC-1α:
- Increases its binding affinity for nuclear respiratory factors
- Enhances transcription of mitochondrial genes
- Triggers mitochondrial biogenesis and improved function
- Coordinates fatty acid oxidation with energy demand
FOXO Transcription Factors: Stress Response Commanders SIRT1's deacetylation of FOXO proteins (particularly FOXO1 and FOXO3) creates a powerful stress resistance network:
- Enhanced nuclear localization and DNA binding
- Increased transcription of antioxidant enzymes
- Upregulation of DNA repair mechanisms
- Activation of autophagy pathways
- Improved cellular stress tolerance
p53: Balancing Protection and Survival SIRT1's deacetylation of p53 at lysine 382 creates a nuanced regulatory mechanism that shifts p53's activity from promoting cell death toward enhancing survival:
- Reduced pro-apoptotic gene expression
- Enhanced DNA repair activity
- Improved cell cycle checkpoint function
- Better genomic stability maintenance
NF-κB: Inflammation Control Center By deacetylating the RelA/p65 subunit of NF-κB, SIRT1 suppresses inflammatory signaling:
- Reduced DNA binding affinity
- Decreased inflammatory gene transcription
- Lower cytokine and chemokine production
- Protection against age-related chronic inflammation
The AMPK Pathway: Energy Sensing and Metabolic Optimization
Dual Activation Mechanisms
Resveratrol activates AMPK through two distinct molecular pathways, creating a powerful energy-sensing enhancement:
Direct AMPK Activation (High-Dose Mechanism) At higher concentrations, resveratrol directly activates AMPK through inhibition of cAMP phosphodiesterases:
- PDE inhibition increases cellular cAMP levels
- Elevated cAMP activates Epac1 (exchange protein activated by cAMP)
- Epac1 signaling leads to AMPK phosphorylation and activation
- Activated AMPK triggers energy conservation and stress resistance
SIRT1-Mediated AMPK Activation (Low-Dose Mechanism) At lower, more physiologically relevant doses, resveratrol activates AMPK through SIRT1:
- Resveratrol activates SIRT1
- SIRT1 deacetylates LKB1 (liver kinase B1)
- Deacetylated LKB1 shows enhanced kinase activity
- Active LKB1 phosphorylates and activates AMPK
The AMPK-SIRT1 Positive Feedback Loop
One of resveratrol's most elegant molecular features is the creation of a self-reinforcing cycle between AMPK and SIRT1:
Phase 1: Initial Activation
- Resveratrol activates SIRT1
- SIRT1 enhances AMPK sensitivity
- AMPK activation begins metabolic changes
Phase 2: NAD+ Enhancement
- AMPK promotes fatty acid oxidation
- Reduced energy-consuming processes
- Increased NAD+ production from enhanced metabolism
Phase 3: Amplified SIRT1 Activity
- Higher NAD+ levels fuel increased SIRT1 activity
- Enhanced SIRT1 further activates AMPK
- Self-reinforcing cycle establishes new cellular equilibrium
This molecular feedback mechanism explains why resveratrol's effects can persist and amplify over time, creating sustained metabolic improvements.
Metabolic Targets of AMPK Activation
Fatty Acid Metabolism Optimization AMPK activation by resveratrol transforms cellular fuel utilization:
- Phosphorylation and inactivation of acetyl-CoA carboxylase (ACC)
- Reduced fatty acid synthesis
- Enhanced fatty acid oxidation
- Improved metabolic flexibility
Glucose Metabolism Enhancement AMPK creates a more efficient glucose handling system:
- Increased glucose uptake in muscle tissue
- Enhanced glycolytic enzyme activity when needed
- Improved insulin sensitivity
- Better glucose tolerance
Mitochondrial Enhancement: Powering Cellular Rejuvenation
Mitochondrial Biogenesis: Creating New Cellular Powerhouses
The convergence of SIRT1 and AMPK signaling on PGC-1α represents one of biology's most potent triggers for mitochondrial enhancement. When resveratrol simultaneously activates both pathways, the combined effect on PGC-1α is dramatic:
Transcriptional Cascade Activation
- Dual SIRT1 deacetylation and AMPK phosphorylation of PGC-1α
- Enhanced PGC-1α binding to nuclear respiratory factors (NRF1/NRF2)
- Increased transcription of mitochondrial genes
- Upregulation of respiratory chain components
- Enhanced mitochondrial DNA replication
Mitochondrial Quality Enhancement Beyond simply creating more mitochondria, resveratrol improves mitochondrial quality:
- Enhanced respiratory chain efficiency
- Improved ATP synthesis capacity
- Better calcium handling
- Reduced oxidative stress production
SIRT3: The Mitochondrial Protector
Resveratrol's activation of SIRT3 provides direct mitochondrial protection through targeted protein deacetylation:
Key SIRT3 Targets in Mitochondria:
- Superoxide dismutase 2 (SOD2): Enhanced antioxidant protection
- Isocitrate dehydrogenase 2: Improved NADPH production for antioxidant systems
- Acetyl-CoA synthetase 2: Enhanced fatty acid oxidation
- Complex I subunits: Improved respiratory chain function
Mitochondrial Quality Control SIRT3 activation enhances several quality control mechanisms:
- Improved mitophagy (removal of damaged mitochondria)
- Enhanced protein folding and stability
- Better mitochondrial dynamics (fusion/fission balance)
- Reduced mitochondrial ROS production
Hormetic ROS Signaling: Beneficial Stress Response
Paradoxically, resveratrol initially increases mitochondrial ROS production at low doses, triggering beneficial adaptive responses:
Hormetic Activation Cascade:
- Mild mitochondrial ROS increase
- Activation of Nrf2 transcription factor
- Enhanced antioxidant response element (ARE) gene expression
- Upregulation of endogenous antioxidant systems
- Net improvement in oxidative stress resistance
This hormetic effect explains why resveratrol's benefits often follow a U-shaped dose-response curve, with optimal effects at moderate rather than very high doses.
Epigenetic Reprogramming: Rewriting the Aging Code
Histone Deacetylation and Chromatin Remodeling
As NAD+-dependent histone deacetylases, sirtuins directly modify chromatin structure, essentially rewriting the cellular aging program through epigenetic mechanisms.
SIRT1-Mediated Histone Modifications:
- H3K9 deacetylation: Creates more condensed, stable chromatin
- H4K16 deacetylation: Alters chromatin accessibility for transcription
- Coordinated gene network regulation: Simultaneous activation/repression of related genes
- Enhanced DNA repair accessibility: Improved access for repair machinery
SIRT6: Chromatin Stability Guardian SIRT6's role in maintaining heterochromatin stability is crucial for genomic integrity:
- Enhanced telomeric chromatin condensation
- Reduced DNA damage at repetitive sequences
- Improved global genome organization
- Protection against age-related genomic instability
Long-Term Epigenetic Effects
Emerging research suggests resveratrol creates lasting epigenetic changes that persist beyond its immediate presence:
DNA Methylation Modulation:
- Potential effects on DNA methyltransferase activity
- Altered methylation patterns at key longevity genes
- Long-term changes in gene expression profiles
Chromatin Remodeling Complex Interactions:
- Enhanced recruitment of beneficial chromatin modifiers
- Improved coordination between different epigenetic systems
- Sustained cellular reprogramming toward youthful states
Anti-Inflammatory Molecular Networks
NF-κB Suppression: Cooling Cellular Fire
Chronic inflammation represents a fundamental driver of aging, and resveratrol's anti-inflammatory mechanisms operate at multiple molecular levels:
Direct NF-κB Inhibition:
- SIRT1 deacetylates RelA/p65 at lysine 310
- Reduced DNA binding affinity and transcriptional activity
- Decreased expression of inflammatory mediators
- Suppression of inflammatory cascade amplification
Upstream Pathway Modulation:
- Inhibition of IκB kinase (IKK) complex
- Reduced IκB protein degradation
- Maintained inhibitory complex formation
- Prevention of NF-κB nuclear translocation
Inflammasome Regulation: Controlling Cellular Danger Signals
Recent research has revealed resveratrol's effects on inflammasome activity:
NLRP3 Inflammasome Suppression:
- Reduced assembly of inflammasome components
- Decreased IL-1β and IL-18 production
- Lower caspase-1 activation
- Reduced cellular danger signal amplification
Mitochondrial Damage Prevention: Through enhanced mitochondrial function, resveratrol reduces:
- Mitochondrial DNA release (a key inflammasome trigger)
- Oxidized mitochondrial components
- Cellular damage-associated molecular patterns (DAMPs)
Cellular Senescence and Autophagy: Clearing the Aged
Senescence Prevention Mechanisms
Cellular senescence represents a crucial aging mechanism, and resveratrol affects this process through multiple pathways:
p53/p21 Pathway Modulation:
- SIRT1 deacetylation shifts p53 activity toward repair rather than senescence
- Reduced p21 expression and cell cycle arrest
- Enhanced DNA repair capacity
- Prevention of irreversible growth arrest
Telomere Protection:
- SIRT6-mediated telomeric chromatin stabilization
- Reduced oxidative damage to telomeres
- Potential enhancement of telomerase activity
- Improved telomere maintenance
Autophagy Enhancement: Cellular Housekeeping
Autophagy represents the cell's primary quality control mechanism, and resveratrol enhances this system through multiple pathways:
FOXO-Mediated Autophagy Gene Expression:
- Increased transcription of autophagy-related genes (ATGs)
- Enhanced expression of lysosomal enzymes
- Improved autophagosome formation
- Better autophagy flux
mTOR Pathway Suppression:
- AMPK-mediated mTOR inhibition
- Removal of autophagy suppression
- Enhanced cellular clearance mechanisms
- Improved protein quality control
Cardiovascular Protection: Molecular Cardioprotection
Endothelial Function Enhancement
Resveratrol's cardiovascular benefits involve sophisticated molecular mechanisms:
Nitric Oxide Pathway Optimization:
- SIRT1 deacetylation activates endothelial nitric oxide synthase (eNOS)
- Enhanced eNOS phosphorylation at activating sites (Ser1177)
- Increased nitric oxide bioavailability
- Improved endothelium-dependent vasodilation
Endothelial Stress Resistance:
- Enhanced antioxidant enzyme expression in endothelial cells
- Improved resistance to oxidative stress
- Better endothelial cell survival under inflammatory conditions
- Maintained endothelial barrier function
Vascular Aging Prevention
Matrix Metalloproteinase (MMP) Regulation:
- Reduced MMP-2 and MMP-9 expression through NF-κB suppression
- Decreased extracellular matrix degradation
- Protection against atherosclerotic plaque instability
- Maintained vascular structural integrity
Smooth Muscle Cell Protection:
- Prevention of pathological smooth muscle cell proliferation
- Reduced migration and phenotypic switching
- Enhanced apoptosis of proliferating cells in atherosclerotic lesions
Neuroprotection: Safeguarding Cognitive Function
Neuronal Survival Enhancement
Resveratrol's neuroprotective mechanisms involve multiple molecular pathways:
BDNF (Brain-Derived Neurotrophic Factor) Enhancement:
- CREB-mediated BDNF gene transcription
- Enhanced neuronal survival signaling
- Improved synaptic plasticity
- Better cognitive function maintenance
Neuronal Stress Resistance:
- Enhanced antioxidant enzyme expression in neurons
- Improved mitochondrial function in brain tissue
- Better calcium homeostasis
- Reduced neuronal apoptosis
Neuroinflammation Suppression
Microglial Modulation:
- Reduced microglial activation and proliferation
- Decreased pro-inflammatory cytokine production
- Enhanced anti-inflammatory signaling
- Better neuroinflammatory resolution
Blood-Brain Barrier Protection:
- Enhanced tight junction protein expression
- Improved barrier integrity
- Reduced inflammatory cell infiltration
- Better neuroprotective environment maintenance
Pathway Integration: The Molecular Symphony
Coordinated Network Effects
The true power of resveratrol lies in the sophisticated integration of multiple molecular pathways:
Central Integration Hub: SIRT1-AMPK-PGC-1α This molecular triad serves as the primary coordination center:
- Energy sensing and metabolic adaptation
- Stress response and cellular protection
- Mitochondrial enhancement and function
- Longevity pathway activation
Secondary Network Effects:
- NF-κB suppression reduces inflammatory stress
- FOXO activation enhances stress resistance
- Autophagy enhancement improves cellular quality
- Epigenetic modifications create lasting changes
Temporal Dynamics of Molecular Activation
Immediate Phase (Minutes to Hours):
- Direct SIRT1 allosteric activation
- Initial AMPK phosphorylation
- Early gene expression changes
- Rapid metabolic adjustments
Intermediate Phase (Hours to Days):
- Protein expression changes
- Mitochondrial enzyme upregulation
- Inflammatory pathway suppression
- Enhanced cellular stress resistance
Long-Term Phase (Days to Weeks):
- Mitochondrial biogenesis completion
- Epigenetic reprogramming stabilization
- Sustained metabolic optimization
- Cellular phenotype transformation
Future Molecular Frontiers
Emerging Mechanisms
Current research continues to reveal new aspects of resveratrol's molecular action:
Circadian Clock Modulation:
- Potential effects on CLOCK and BMAL1 proteins
- Influence on metabolic circadian rhythms
- Connection between timing and longevity pathways
Microbiome-Host Molecular Communication:
- Gut microbiome metabolite production
- Molecular signaling between microbes and host cells
- Systemic effects of microbial resveratrol metabolism
Tissue-Specific Molecular Programs:
- Different molecular responses in various tissues
- Organ-specific pathway activation patterns
- Potential for targeted therapeutic approaches
Implications for Therapeutic Development
Understanding these molecular mechanisms has profound implications:
Next-Generation Compound Development:
- More potent, selective sirtuin activators
- Pathway-specific modulators
- Improved bioavailability formulations
Combination Therapy Strategies:
- Rational combinations with other longevity compounds
- Synergistic pathway activation approaches
- Personalized intervention protocols
Biomarker Development:
- Molecular markers of pathway activation
- Real-time monitoring of resveratrol effects
- Optimization of dosing and timing
Conclusion: A Molecular Masterpiece of Longevity
Resveratrol's molecular mechanisms represent perhaps the most sophisticated example of how a single natural compound can orchestrate comprehensive cellular rejuvenation. From its initial discovery as a simple SIRT1 activator to our current understanding of its complex multi-pathway effects, resveratrol has revealed the intricate molecular machinery that governs cellular aging and protection.
The compound's ability to simultaneously enhance energy metabolism, strengthen cellular defenses, improve quality control mechanisms, and suppress damaging processes creates a remarkable molecular symphony that addresses aging at its most fundamental levels. Each pathway reinforces the others, creating a coordinated cellular response that promotes longevity and healthspan.
Perhaps most remarkably, resveratrol has served as a molecular Rosetta Stone, helping scientists decode the language of longevity and understand how different aspects of aging are interconnected at the cellular level. This knowledge continues to drive advances in longevity research and our understanding of how we might enhance human healthspan through targeted molecular interventions.
As we continue to unravel the full complexity of these molecular networks, we gain not just insights into a remarkable compound, but a deeper understanding of the fundamental processes that govern aging itself. In this cellular symphony, every molecule plays its part, every interaction matters, and every discovery brings us closer to understanding—and potentially mastering—the aging process at its most basic level.
https://aging-bio.com/search_index.en.json$MATCHES more matches